effective strategies for rescue studies
protocol assumptions verification
study population size validation
statistical analysis plans reviews
statistical calculations validation
data quality audits
For the last 20 years we have been providing the highest quality standards and effective support to our partners and their realization of clinical trials and observation studies.
Quality and security
Realization of projects in accordance with applicable industry standards (including the GCP and FDA 21 CFR Part 11). Guaranteed high quality and data security confirmed by ISO 9001 and 27001 certificates and a set of internal SOP procedures.
Full service support
Professional realization of each stage of the project in cooperation with an interdisciplinary team of specialists experienced in the realization of international studies.
Newest technologies
Modern and effective IT tools that allow flexible adaptation to the specific requirements of each study. Freedom of integration with external data sources, such as mobile devices, modern medical devices or medical systems databases.
News
CRO Services
2KMM CRO is a trusted provider of comprehensive solutions and services supporting management and execution of research projects for pharmaceutical industry, biotechnology, medical devices and healthcare.
As a contract research organization we offer substantial expertise in the following areas of clinical research:

GoResearch™.live
GoResearch™.live is a fully validated, next-generation Electronic Data Capture (EDC) platform tailored for modern clinical trials and observational studies. It offers fast, no-code setup of customizable electronic Case Report Forms (eCRFs), robust real-time data validation, and full compliance with FDA 21 CFR Part 11, GCP, and GDPR. Designed for flexibility, it supports all study models—from traditional to hybrid and decentralized. Its secure API enables seamless integration with external systems, enhancing studies with electronic source data (eSource) and Real World Data (RWD). With intuitive tools and real-time oversight, GoResearch™.live streamlines data collection and boosts study efficiency.
more ...GoResearch™ App
GoResearch™ App is a dedicated patient or clinician mobile application to support study data collection from electronic questionnaires (e.g. ePRO or eCOA) or connected mHealth devices. Seamlessly integrated with the eCRF platform provides instant data transfer to the study database and direct communication in a form of study alerts and/or reminders. Available for both Android and iOS systems.
GoInsights™
GoInsights™ platform gives you the freedom you need to create a data collection or presentation tool that suits your goal best. ePRO, eCOA, eIC or investigators training can be built using various data entry options and enriched with multimedia content. Hardware agnostic and fully responsive delivers equally great user experience to either desktop or mobile devices users. Can be run in a standalone or integrated with the GoResearch™ platform mode.
more ...
project management
study documentation development
and management
site and patient recruitment
study monitoring
site management
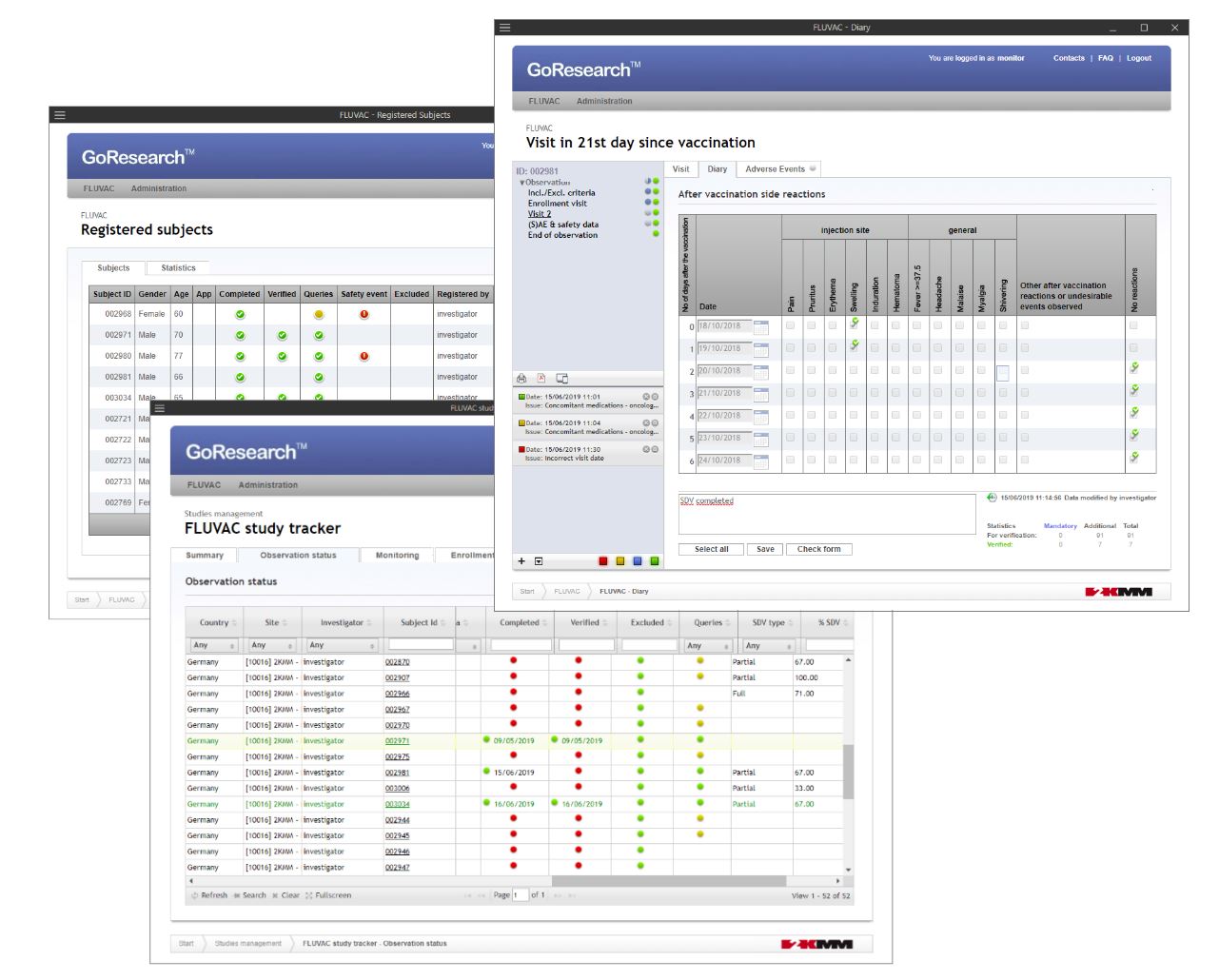
eCRF design and development
data management plans
data validation plans
query management
medical coding
protocol development support
statistical analysis plans
statistical design and analysis
final and interim study reports
randomization and blinding

protocols
investigator brochures
narratives
clinical study reports (CSR)
manuscripts
abstracts

adverse events monitoring
safety database maintenance
SAE/SUSAR/USADE reporting
safety management plans
safety guidelines
case narratives writing
periodic reports





